Genetically encoded biosensors for neuronal imaging
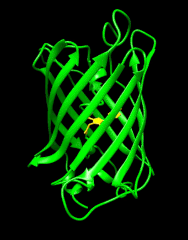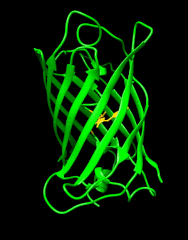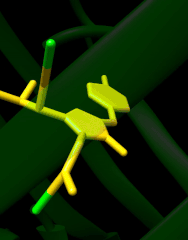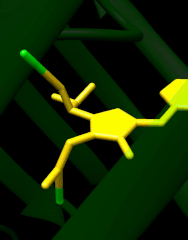
Biosensors that modulate their fluorescence intensity in response to changes in ligand concentration are powerful tools for visualizing large-scale brain activity in model organisms at high resolution. My research involves using green fluorescent protein (GFP) and other colored variants of GFP to develop genetically encoded biosensors that modulate their fluorescence intensity or color in response to a specific biochemical change. In addition, I focus on broadening the spectrum of available colors, and developing biosensors with varying colors to enable multiplex, large-scale recording of neural activity in the brain. These tools help tackle challenging questions, and understand how neurons transform inputs into outputs in the brain.
Genetically encoded biosensors for neurotransmitters, ions, and metabolites




Building on the previous generation of the state-of-the-art SF-iGluSnFR scaffold (itself an improvement over earlier versions), Janelia Researchers, led by Abhi Aggarwal and Kaspar Podgorski, have further improved specificity for synaptic versus extrasynaptic glutamate. iGluSnFr3 variants are more sensitive, faster, and better localized for synaptic glutamate response than its predecessors.
Potassium ion (K+) homeostasis and dynamics play critical roles in biological activities. GINKO1 (green indicator of K+ for optical imaging) is a single fluorescent protein-based K+indicator constructed by insertion of Kbp into enhanced green fluorescent protein (EGFP). GINKO1, in conjunction with red fluorescent Ca2+indicator, enable dual-color imaging of K+ and Ca2+ dynamics in neurons and glial cells.
To enable investigations of the emerging roles of cell-to-cell shuttling of L-lactate, we have developed an intensiometric green fluorescent genetically encoded biosensor for extracellular L-lactate. We demonstrate that this biosensor, designated eLACCO1.1, enables minimally invasive cellular resolution imaging of extracellular L-lactate in cultured mammalian cells and brain tissue.
Here we design and optimize a genetically encoded fluorescent indicator, iAChSnFR, for the ubiquitous neurotransmitter acetylcholine, based on a bacterial periplasmic binding protein. iAChSnFR shows large fluorescence changes, rapid rise and decay kinetics, and insensitivity to most cholinergic drugs. iAChSnFR revealed large transients in a variety of slice and in vivo preparations in mouse, fish, fly and worm.
Detecting Spontaneous Release of Glutamate using iGluSnFR3


DIV 12 Primary Cortical Neuronal Culture
10uM TTX, 75mM sucrose
Widefield; NA 1.4, 60X
F0 subtracted, 12.5Hz
[GECIs] Genetically encoded calcium indicators




In an effort to sustain the steady progression toward ever-improved GECIs, we undertook the development of a new GECI based on the bright monomeric GFP, mNeonGreen (mNG). mNG-GECO1 is a promising next-generation GECI that could inherit the mantle of GCaMP and allow the steady improvement of GECIs to continue for generations to come.
To take advantage of inexpensive and powerful industrial lasers for monitoring neuronal activity, high-performance biosensors that excite at wavelengths above 1,000 nm are needed. We therefore set out to develop jYCaMP1 that outperforms its parent in mice and flies at excitation wavelengths above 1,000 nm and enables improved two-color calcium imaging with red fluorescent protein-based indicators.
Genetically encoded calcium ion (Ca2+) indicators (GECIs) are widely-used molecular tools for functional imaging of calcium dynamics and neuronal activities on a single cell level. Here we report the design and development of two new far-red fluorescent GECIs, FR-GECO1a and FR-GECO1c, based on the monomeric far-red fluorescent protein mKelly.
We report an intensiometric, near-infrared fluorescent, genetically encoded calcium ion (Ca2+) indicator (GECI) with excitation and emission maxima at 678 and 704 nm, respectively. This GECI, designated NIR-GECO1, enables imaging of Ca2+ transients in cultured mammalian cells and brain tissue with sensitivity comparable to that of currently available visible-wavelength GECIs.
Detecting Neuronal Activity using a Blue-Shifted Ca2+ Indicator
